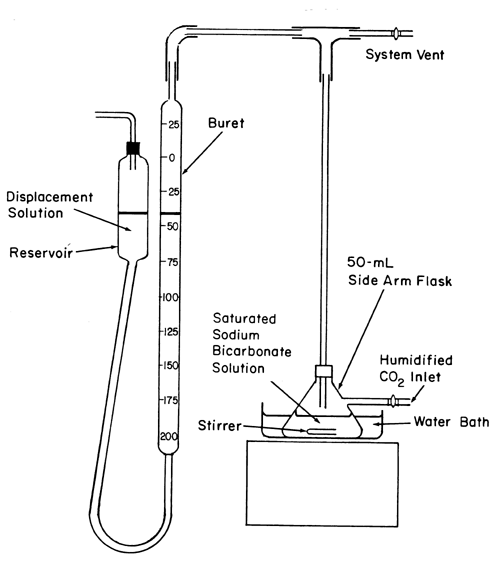
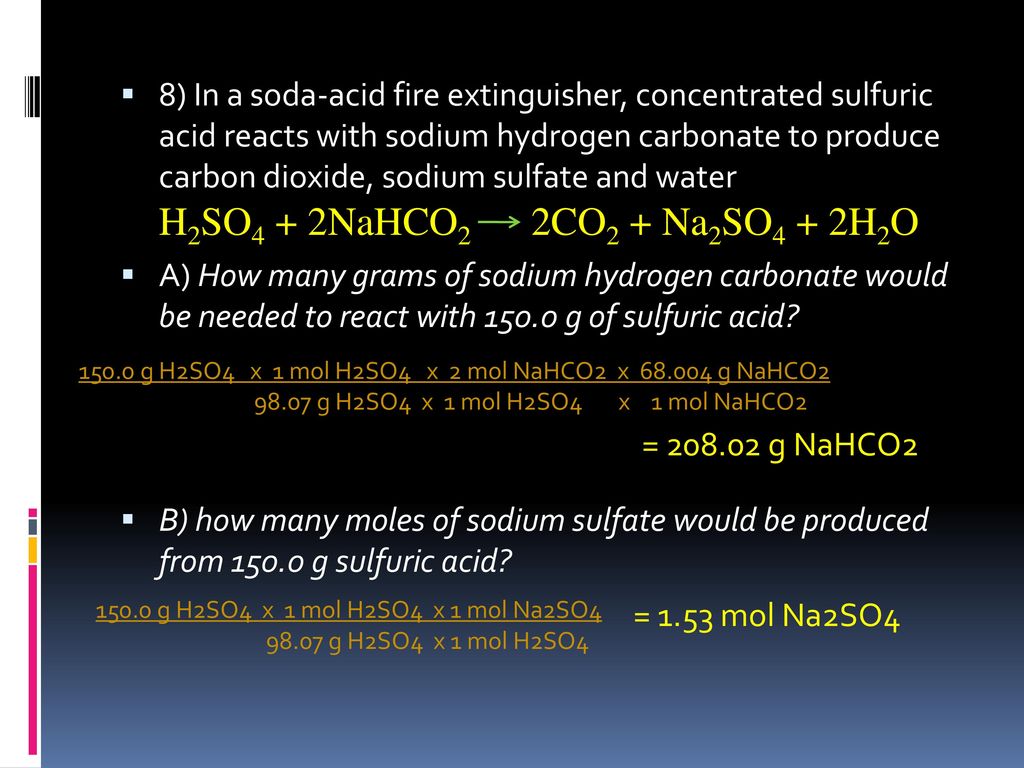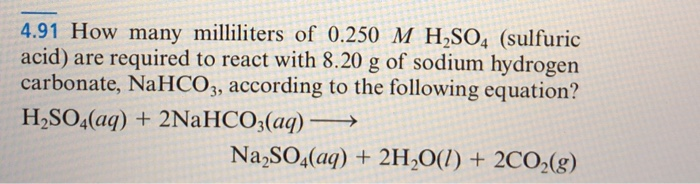Question Video: Identifying the Volatile Compound in a Reaction for Volatilization Gravimetry | Nagwa

Question Video: Determining the Concentration of Sulfuric Acid Via Titration with Sodium Carbonate | Nagwa

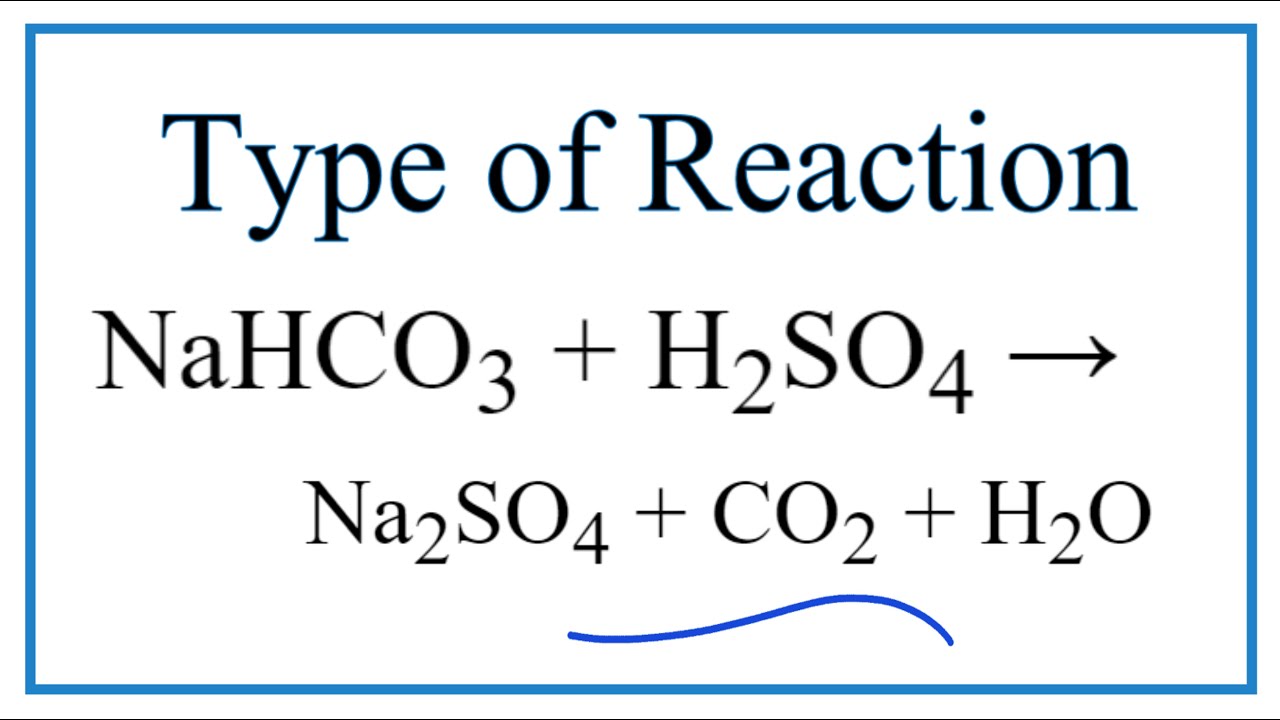
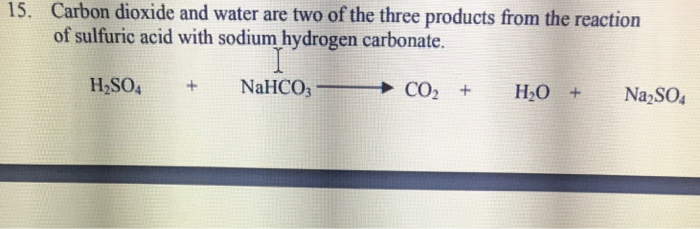
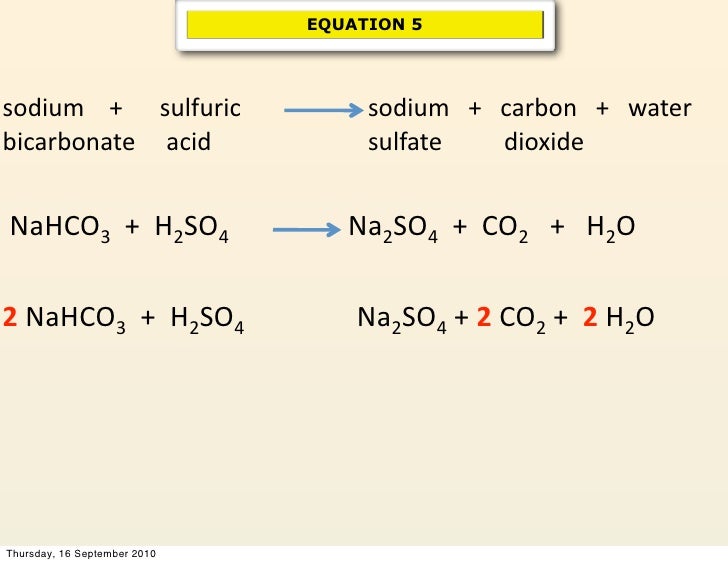
balance the equation Sodium bicarbonate + Sulphuric acid = Sodium sulphate + Water + Carbon dioxide. - Brainly.in

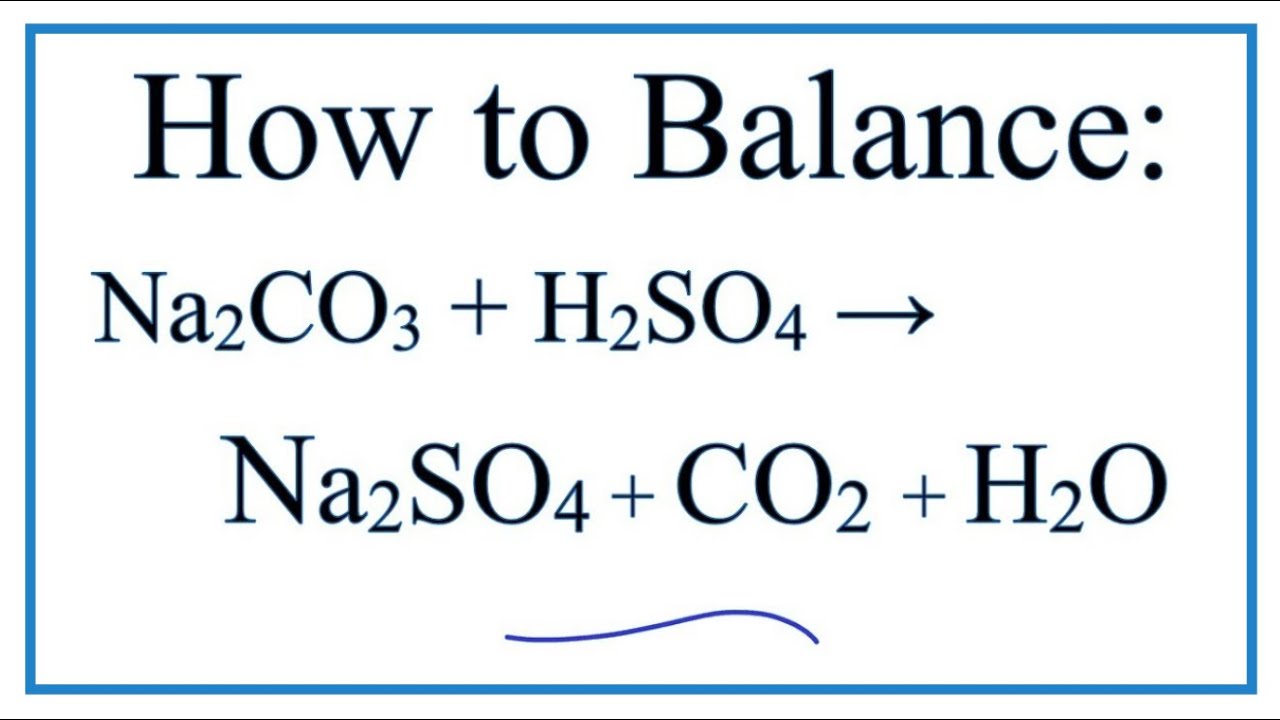
NaHCO3+H2SO4=Na2SO4+H2O+CO2 balance the equation @mydocumentary838. nahco3+ h2so4=na2so4+h2o+co2 - YouTube
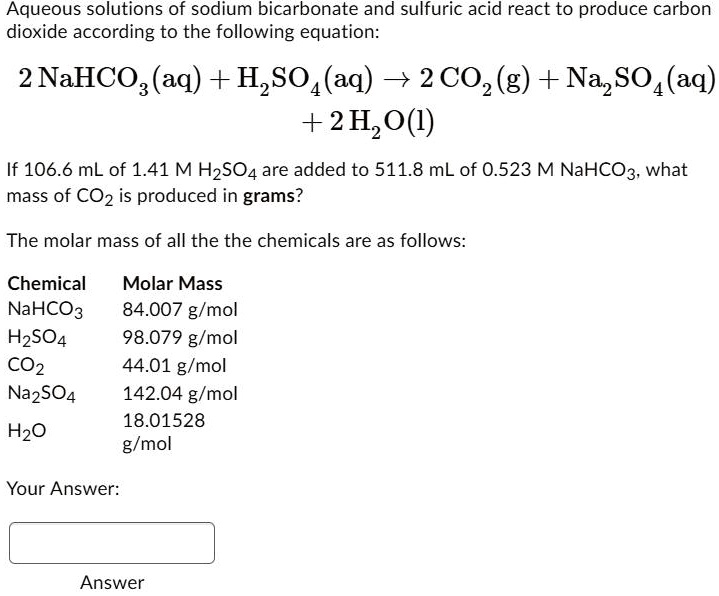
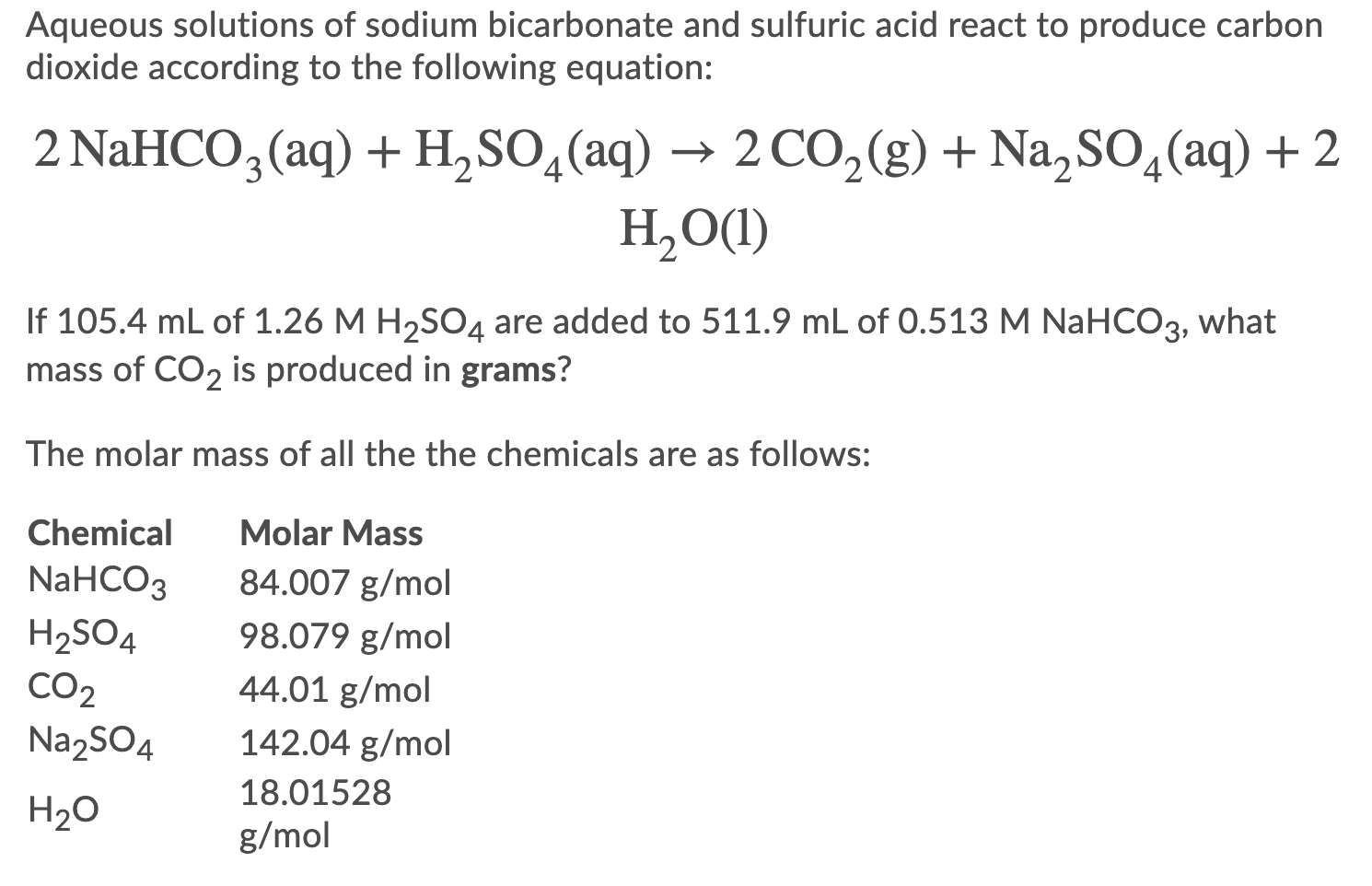
SOLVED: Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: 2 NaHCO3(aq) + H,SO4(aq) = 2 C02(g) + Na2 SO4(aq) +2H20(1) If 106.6

What are Acids? An acid is any compound that yields hydrogen ions (H + ) or hydronium ions (H 3 O + ) when dissolved in water. Hydronium ions are really. - ppt download

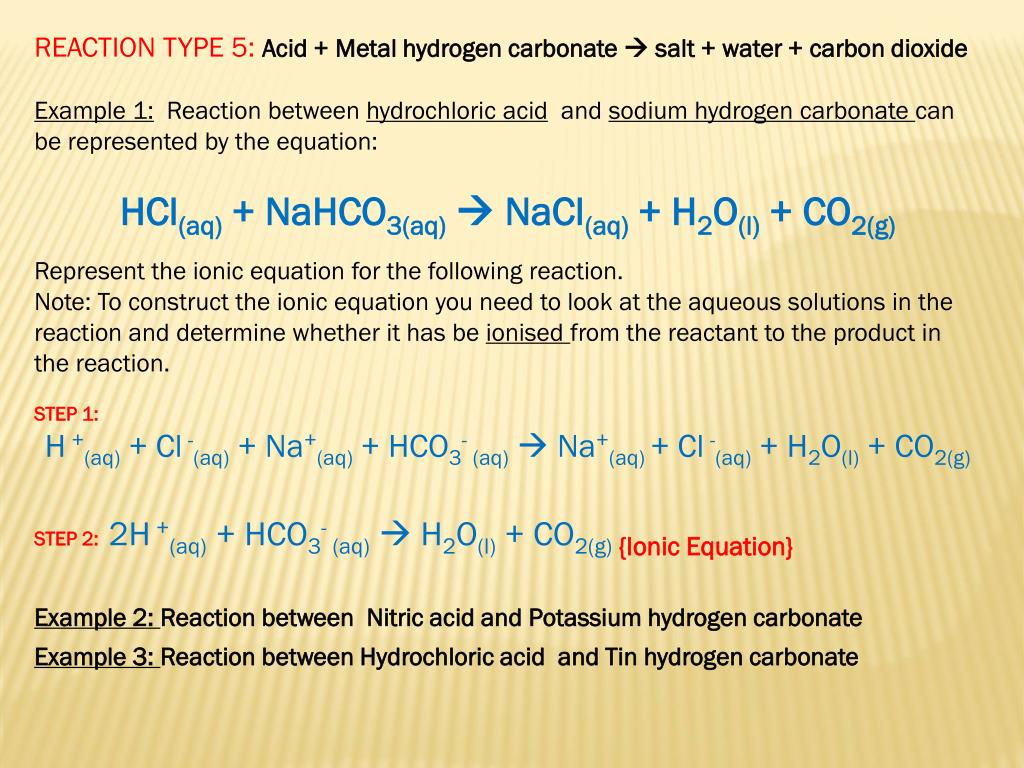
Write the balanced ionic equation for the reaction of sodium bicarbonate with sulphuric acid - YouTube

A student dissolves 3g of impure potassium hydroxide in water and makes the solution up to 250cm3. The student then takes 25.0cm3 of this solution and. - ppt download

The graph of moles of sodium hydrogen carbonate versus moles of sulfuric acid produce a linear graph with the equation y = 2x - 2E-17. The molar mass of sodium hydrogen carbonate
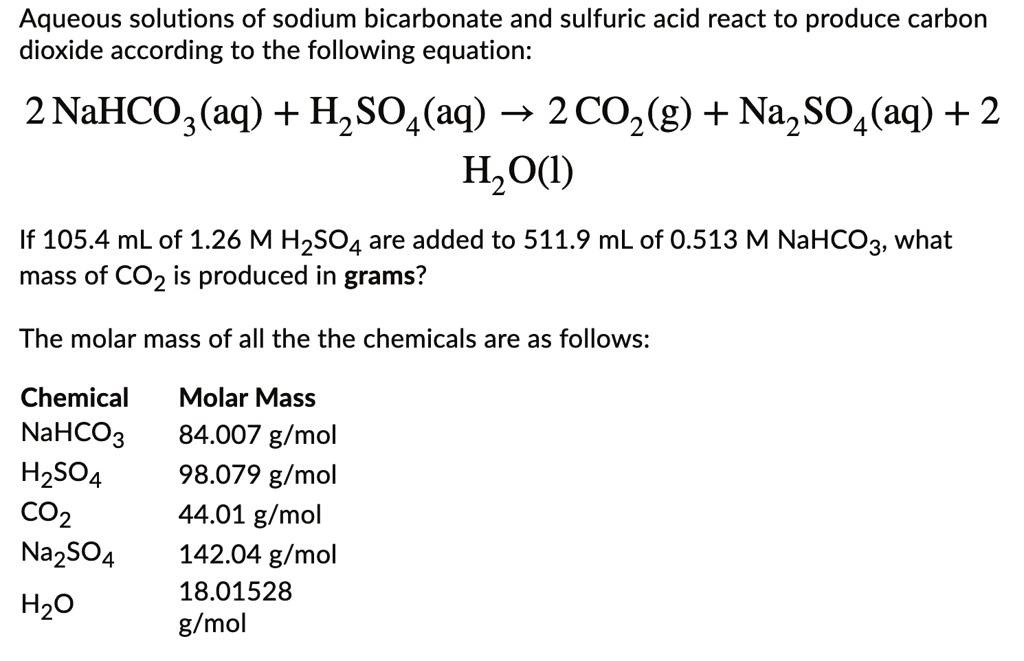
SOLVED: Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: 2 NaHCOs(aq) + H,SO4(aq) 3 2CO2(g) + NazSO4(aq) + 2 HzO() If 105.4