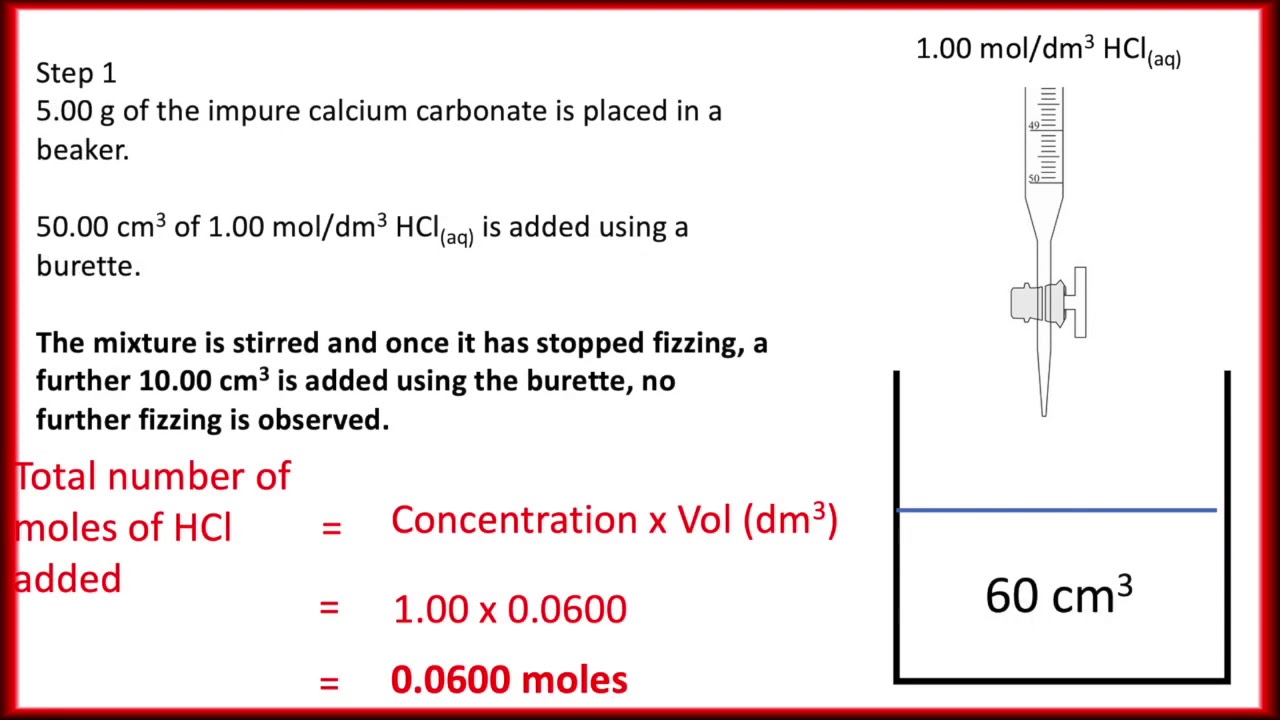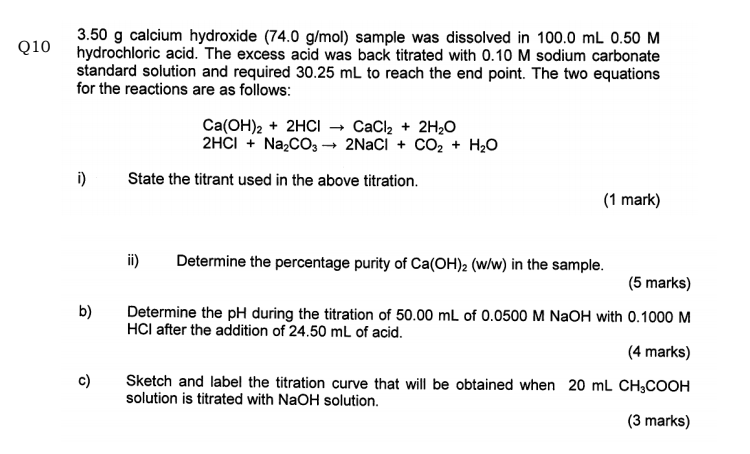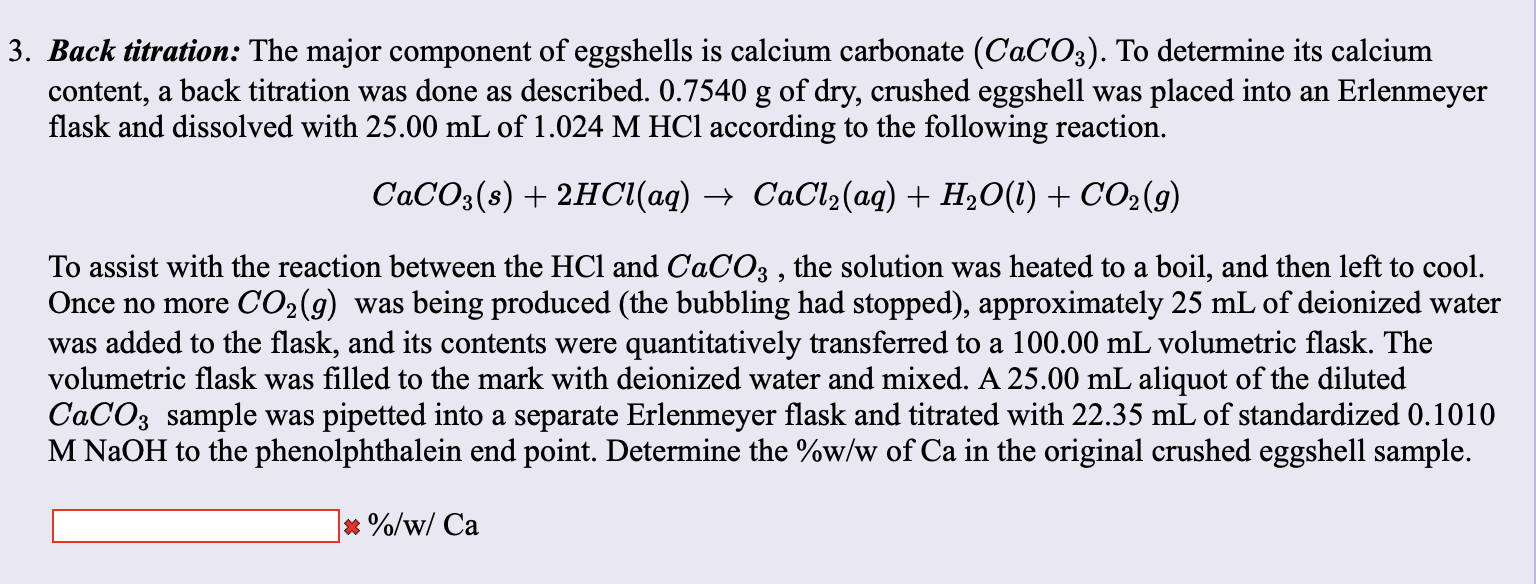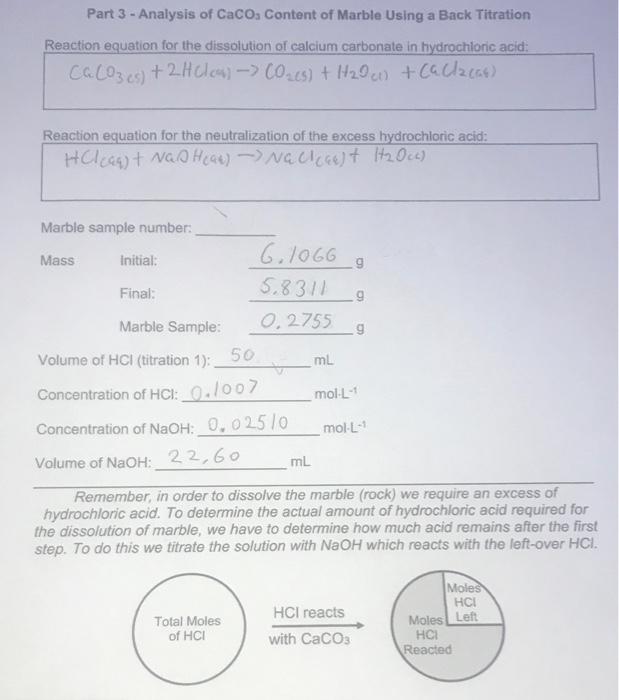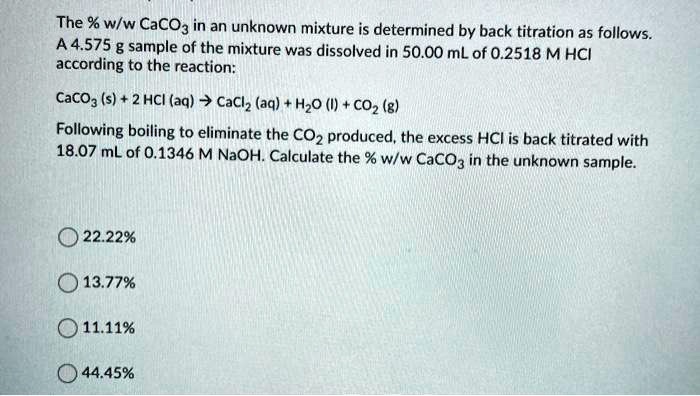
SOLVED: The % w/w CaCO3 in an unknown mixture is determined by back titration as follows: A 4.575 g sample of the mixture was dissolved in 50.00 mL of 0.2518 M HCl

Back-Titration F22 - Copy - Back-Titration of a Commercial Antacid Stephanie R. Geggier New York - Studocu

The relationship between the log amount of pure calcium carbonate and... | Download Scientific Diagram

DOC) OBJECTIVE To determine the calcium carbonate content in eggshell | Sharifah Hana - Academia.edu


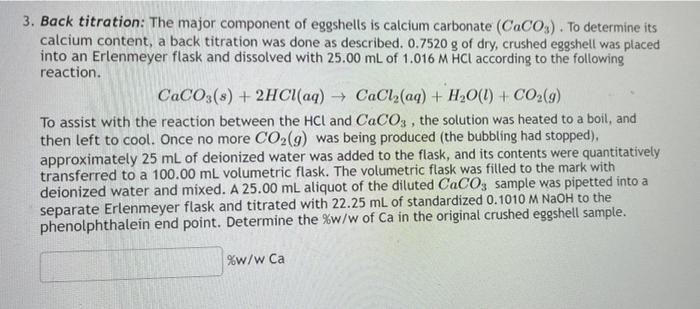




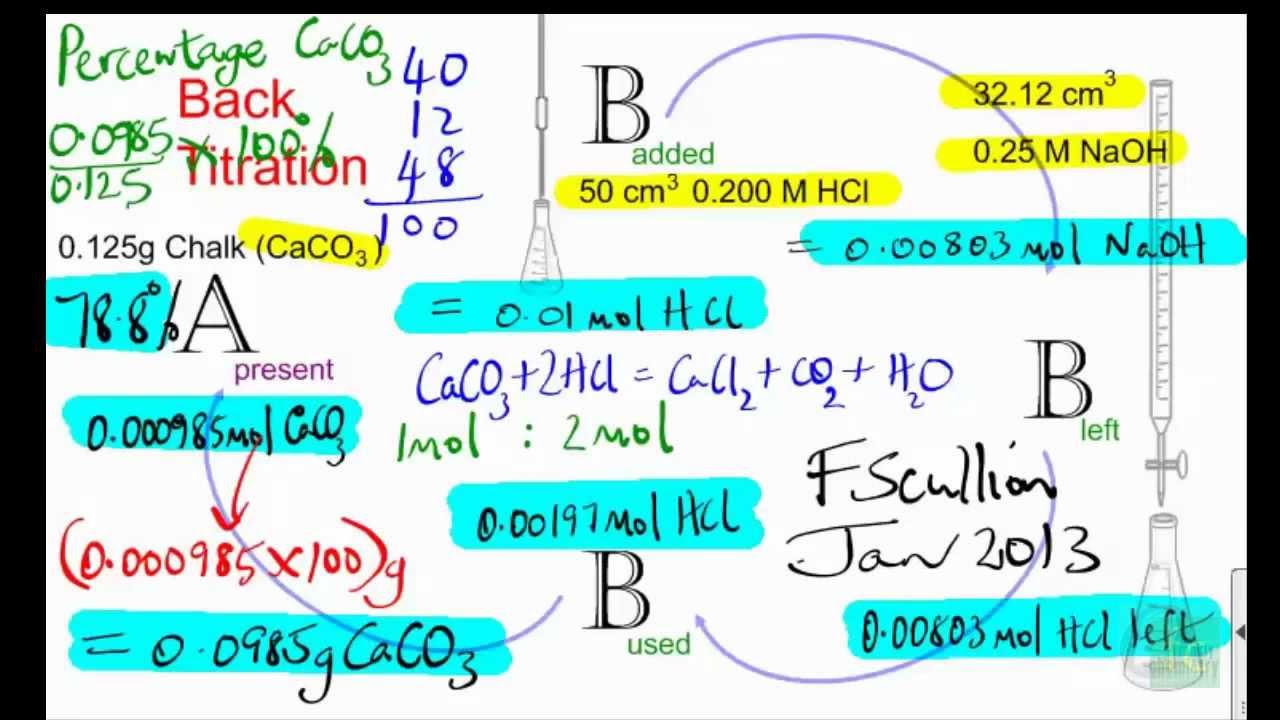
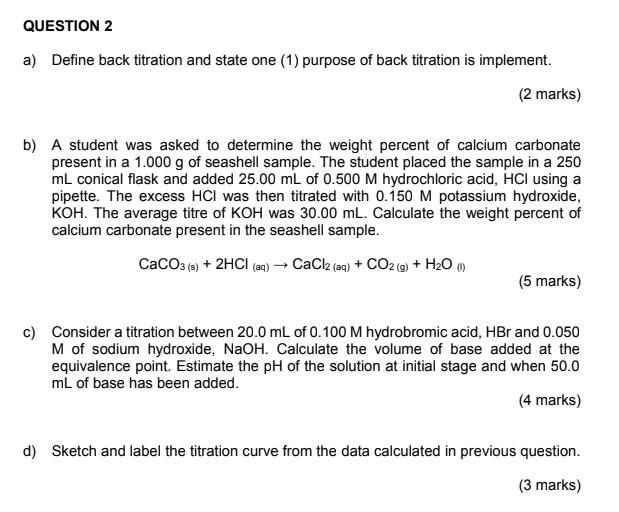







![Lab: Back titration to determine the % CaCO3 in eggshell DATA COLLECTION [IB CHEMISTRY] - YouTube Lab: Back titration to determine the % CaCO3 in eggshell DATA COLLECTION [IB CHEMISTRY] - YouTube](https://i.ytimg.com/vi/fH-RzzDN0Lg/hq720.jpg?sqp=-oaymwEhCK4FEIIDSFryq4qpAxMIARUAAAAAGAElAADIQj0AgKJD&rs=AOn4CLAUKQdGwq7plYa7h8dYUpGwcEOhrA)
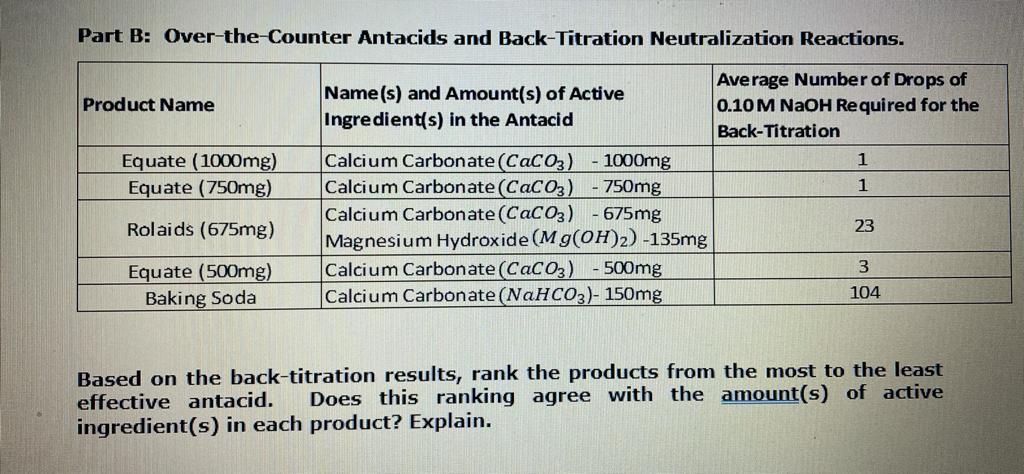
![Lab: Back titration to determine the % CaCO3 in eggshell DATA COLLECTION [IB CHEMISTRY] Lab: Back titration to determine the % CaCO3 in eggshell DATA COLLECTION [IB CHEMISTRY]](https://i.ytimg.com/vi/DjBXw7toD_0/maxresdefault.jpg)