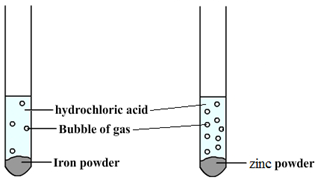
2:15 understand how metals can be arranged in a reactivity series based on their reactions with: water and dilute hydrochloric or sulfuric acid - TutorMyself Chemistry

Sulfuric Acid LO: Outline uses and reactions involving Sulfuric Acid Starter: What is an acid? - ppt download

Effect of sulfuric acid concentration on iron dissolution and final pH.... | Download Scientific Diagram
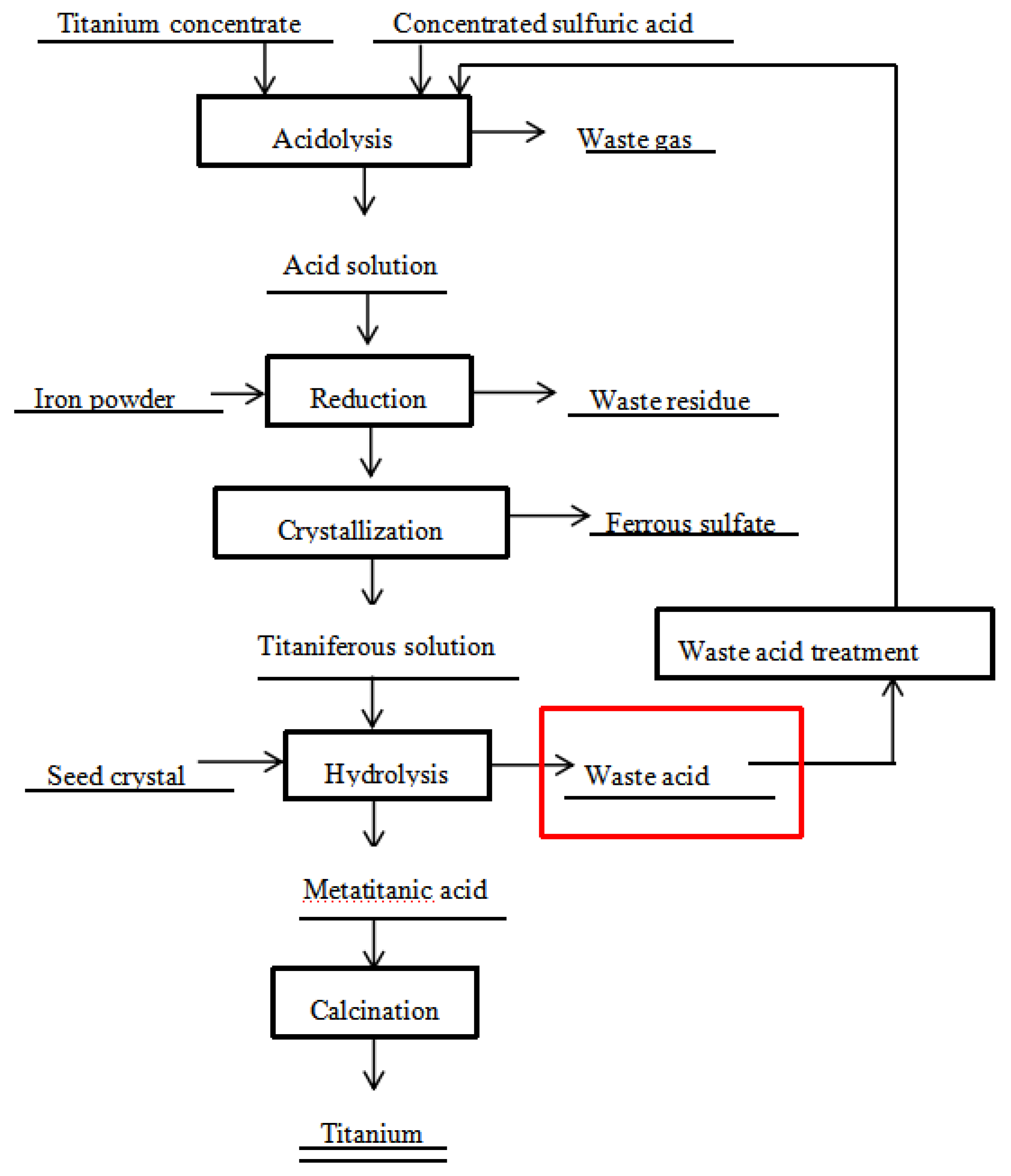
Metals | Free Full-Text | Preparation of Doped Iron Phosphate by Selective Precipitation of Iron from Titanium Dioxide Waste Acid
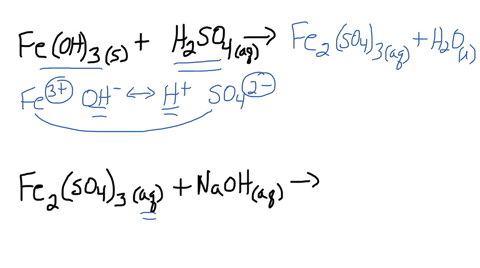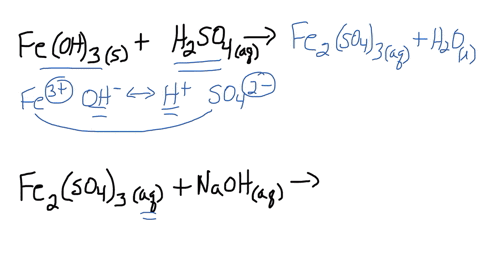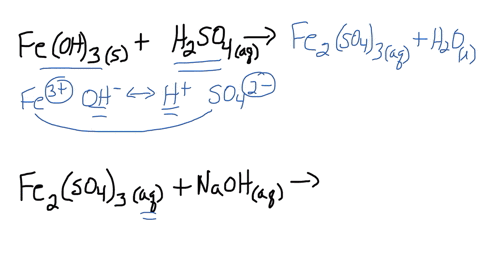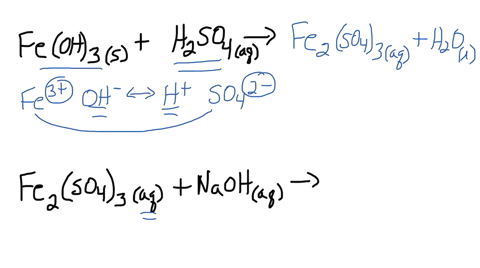
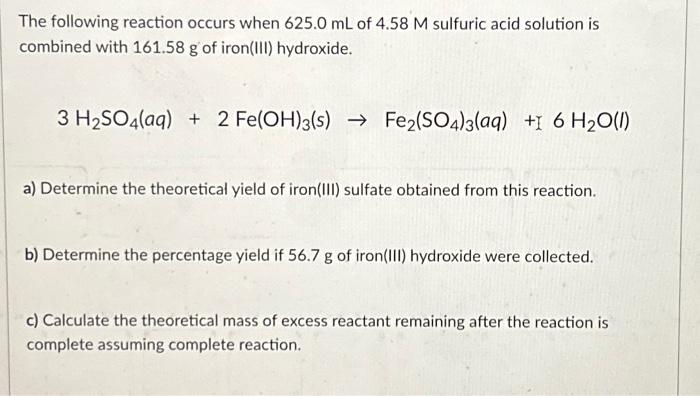
SOLVED: Iron(III) sulfate is made in industry by the neutralization reaction between solid iron(III) hydroxide and aqueous sulfuric acid. The iron(III) sulfate is then added with sodium hydroxide to municipal water in

Corrosion of iron and nickel based alloys in sulphuric acid: Challenges and prevention strategies - ScienceDirect

Fe2O3+H2SO4=Fe2(SO4)3+H2O Balanced Equation|| Balanced equation for Iron iii oxide and Sulfuric acid - YouTube
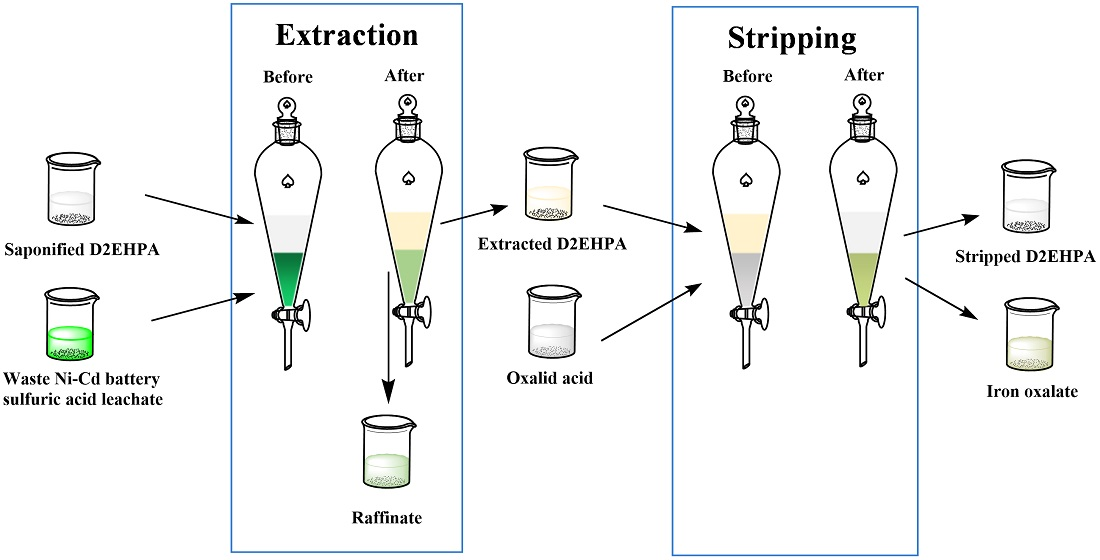
Separations | Free Full-Text | High-Value Recovery of the Iron via Solvent Extraction from Waste Nickel-Cadmium Battery Sulfuric Acid Leachate Using Saponified D2EHPA