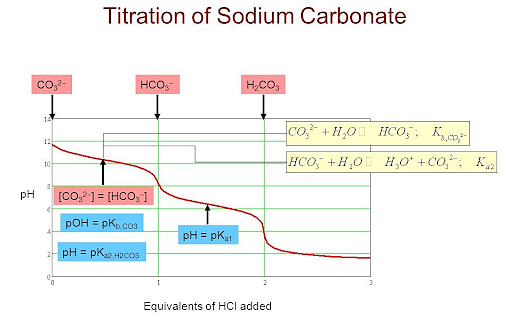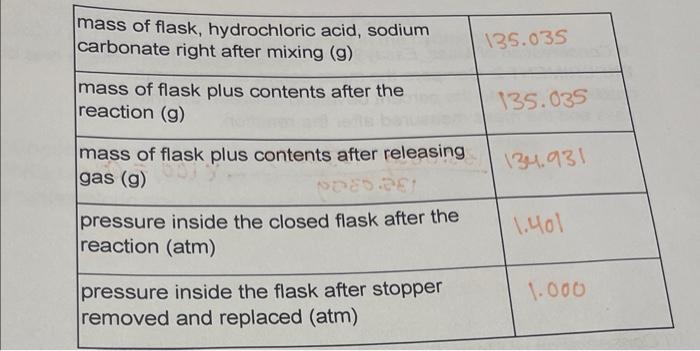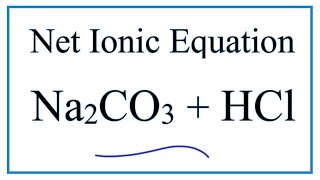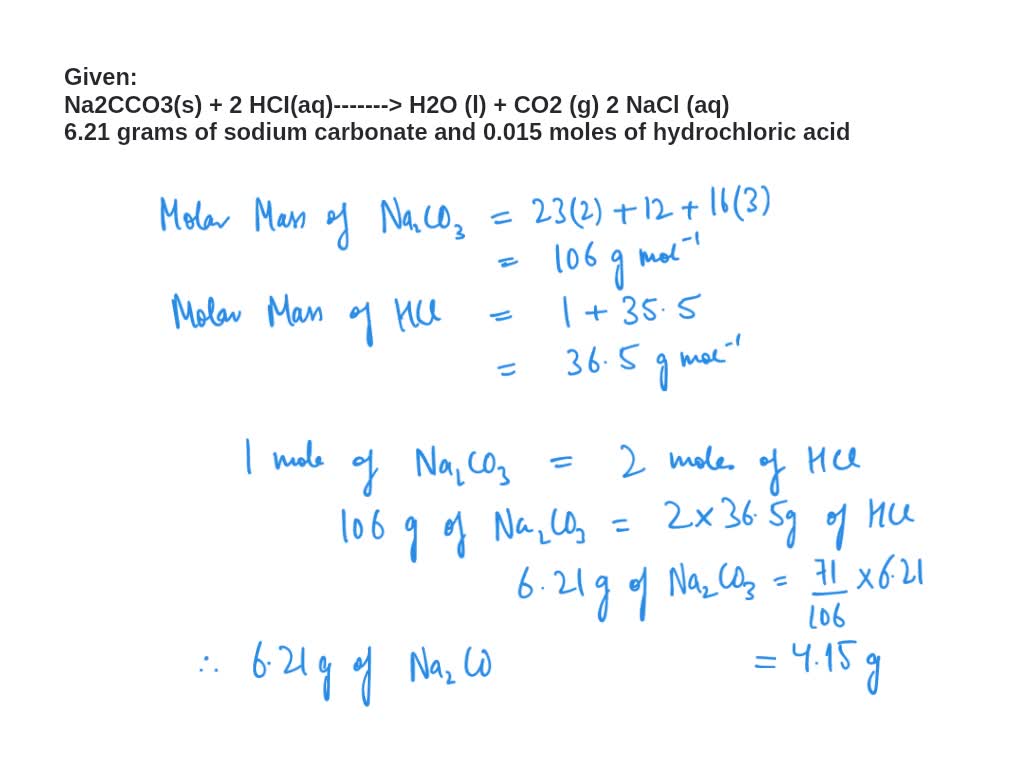
SOLVED: Use this balanced equation to solve the following problem: Na2CO3 (s) + 2 HCl (aq) â†' H2O (l) + CO2 (g) + 2 NaCl (aq). 6.21 grams of sodium carbonate are
What is the reaction between baking soda and hydrochloric acid? How can this reaction be used practically? - Quora

Write a balanced chemical equation the reaction of calcium carbonate and dil. hydrochloric acid.{ CaCO }_{ 3 }+2HClrightarrow { CaCl }_{ 2 }+{ CO }_{ 2 }+{ H }_{ 2 }O{ CaCO }_{
When a powder treated with dilute HCl gas was produced when a lighted matchstick is brought near it, the flame was put off and the gas stopped burning. What is the powder