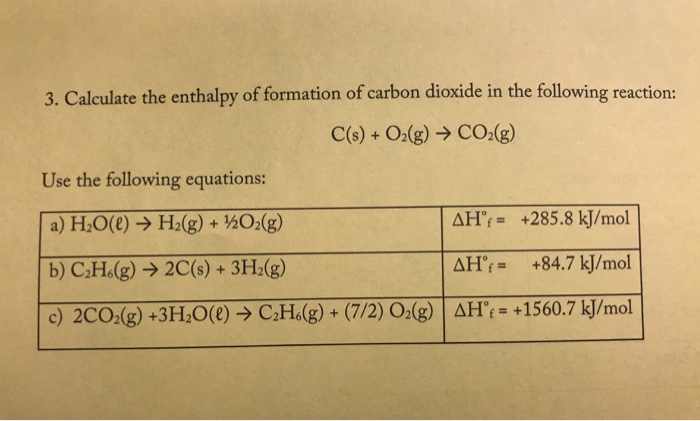
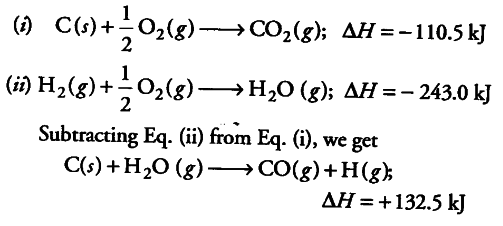![PDF] Prediction of Enthalpy of Formation in the Solid State (at 298.15 K) using Second-Order Group Contributions. Part 1. Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds | Semantic Scholar PDF] Prediction of Enthalpy of Formation in the Solid State (at 298.15 K) using Second-Order Group Contributions. Part 1. Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c766ff3889423f02f154fa43603214c3fec1f15d/4-Table1-1.png)
PDF] Prediction of Enthalpy of Formation in the Solid State (at 298.15 K) using Second-Order Group Contributions. Part 1. Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds | Semantic Scholar
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110, 398, 81, 97 KJ/lol respectively. Find value of H for the reaction. N2O4(g) +3CO(g) — >N20(g)+3CO2(g)

Table 5 from Prediction of Enthalpy of Formation in the Solid State (at 298.15 K) using Second-Order Group Contributions. Part 1. Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds | Semantic Scholar

The enthalpies of formation of carbon nanomaterials as a key factor for understanding their structural features - Physical Chemistry Chemical Physics (RSC Publishing)

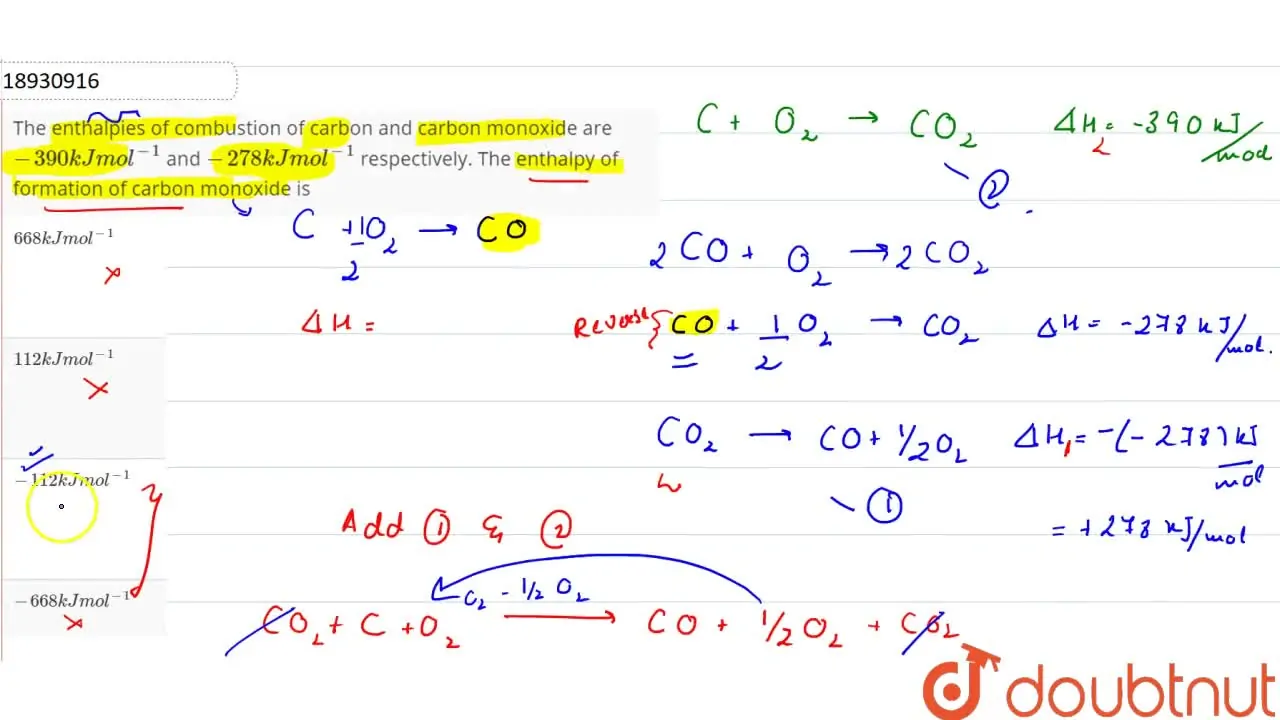
The heat of the combustion of graphite and carbon monoxide respectively are 393.5 kJ mol1 and 283 kJ mol ^{ -1 }. Thus heat of formation of carbon monoxide in kJ mol ^{ -1 } is:-110.5+110.5-55.25None of these